
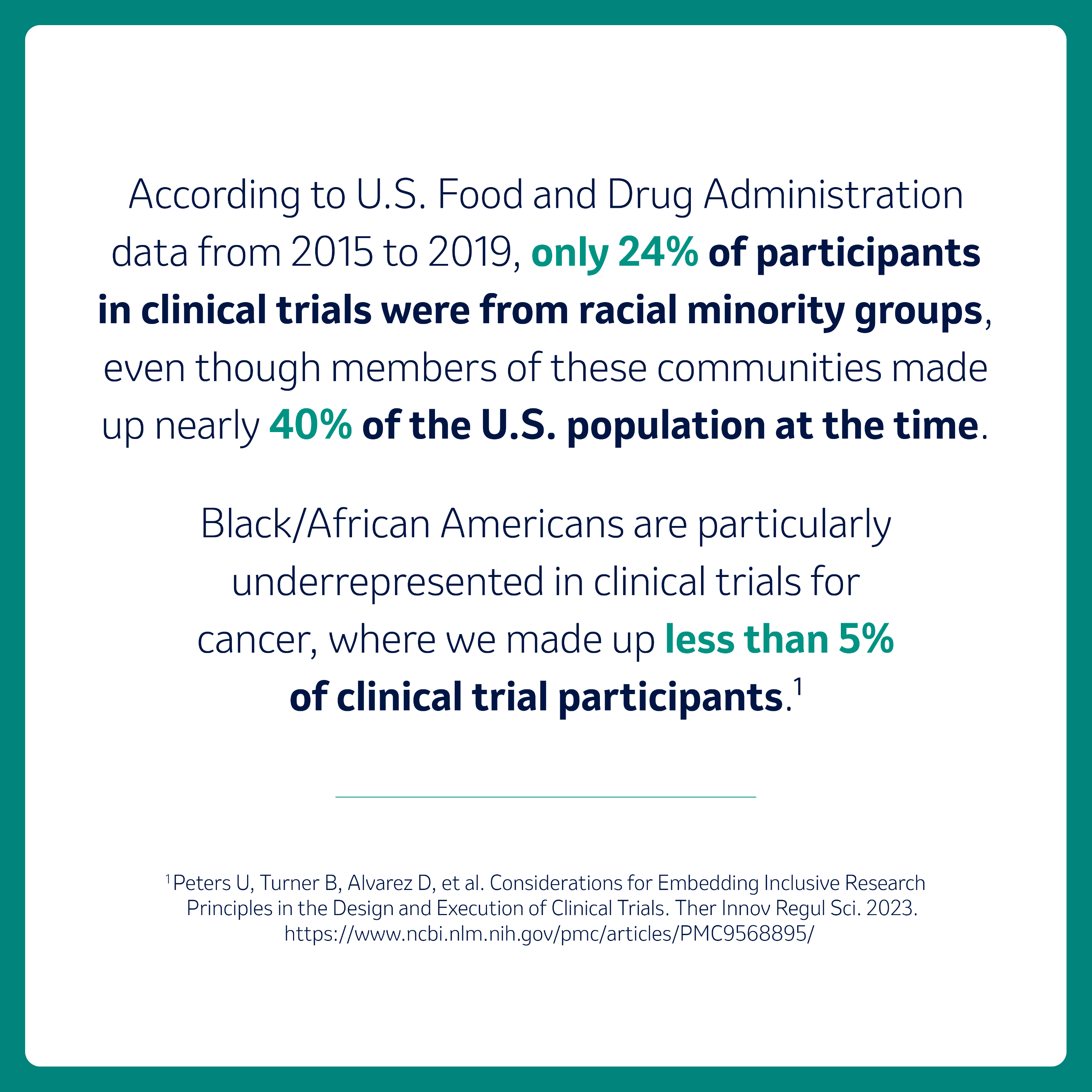
For those of us who have not yet participated in a clinical trial, it may feel like unfamiliar territory. However, understanding what clinical trials are and why they’re important for our community is critical. Clinical trials allow researchers to understand whether investigational medicines may work for people with different health conditions. It is important that people of different ages, genders, race, ethnicities, and socioeconomic backgrounds be represented in clinical research to ensure the data generated reflects the diversity of the population. However, there are many groups of people that have been historically underrepresented in clinical research, including the Black community.
If you ask Dr. Luther T. Clark, Executive Director, Patient Innovation & Engagement, Global Medical and Scientific Affairs at Merck, about the importance of clinical trials he’d say that clinical trials are “a cornerstone of progress in healthcare, helping to determine the safety and effectiveness of investigational medicines.” While prostate cancer patient advocate, Euvon Jones explains, “If you’re fortunate enough to be healthy, the term ‘clinical trial’ probably isn’t part of your everyday vocabulary. You don’t look it up or research it because it doesn’t seem relevant. It’s not that it’s taboo—it just doesn’t feel necessary when you’re well.”
They both agree that clinical trials are most impactful when they involve a diverse group of people. Dr. Clark says, “Without diverse representation, the results are not as applicable to the broader population, nor will they help address healthcare disparities, such as mortality, life expectancy or lack of access to care, which have persisted for far too long.”
Real Life Representation

“When I first heard the words ‘clinical trial,’ I didn’t know a lot about them and I knew I had some homework to do,” says Euvon. “I had heard the term before and never really understood it.” But he had a great connection with his oncologist whose father also had prostate cancer. “Once I learned that a clinical trial could be part of my journey, I trusted my provider to prioritize my health and quality of life. Knowing that I could trust my provider made all the difference. My oncologist could relate to my situation on a personal level. What made me even more confident was the way my oncologist involved my entire family in the process. She gave my wife and me detailed packets of information, and we made copies for our children. We all read the material together and prayed over it. That prayer support was crucial because I knew I had a big decision to make.”
Looking back at his diagnosis with Stage 4 metastatic prostate cancer, nearly 14 years ago, he says he might have been upset if you’d asked him how he felt about it at the time. “But now,
having walked this journey, I wouldn’t trade this experience for anything in the world. It’s been a profound and enlightening experience,” Euvon says. “This journey has also underscored the importance of leadership and advocacy, and I’m glad to share my story and help uplift others who may be going through a similar experience.”
Knowledge is Power
Euvon recalls the early part of his journey consisted of learning as much as he could about clinical trials and understanding his diagnosis. He found that talking with his doctor, loved ones and community helped him feel supported throughout his journey. Dr. Clark agrees that educating yourself about clinical trials is critical: “It’s crucial that people fully understand what clinical trials are all about—their purpose, benefits, and risks. When
people are well-informed and feel confident asking questions, they can make decisions about whether or not to participate in a clinical trial.”
A common misconception surrounding clinical trials is the idea that participating means you’re a “guinea pig.” “In our community, there continues to be lingering mistrust of clinical
trials, due to historical injustices in medical research, such as the Tuskegee Study,” says Dr. Clark. “While some research studies in the past were not conducted in the most appropriate ways, modern clinical trials are conducted with strict guidelines to help protect and respect patients.”
Advancing Health Equity
Dr. Clark and his team at Merck have made diversity and equity a foundational part of their research by creating detailed diversity action plans. “These strategic frameworks outline
specific steps to ensure underrepresented communities are aware of and actively included in our studies. They help to promote inclusivity at every stage of the process,” says Dr. Clark.
The team at Merck also focuses on listening to what patients and providers have to say and incorporating their feedback when designing clinical trials. For example, they heard some people were having challenges with accessing clinical trial locations. To help address this, Merck has brought research sites closer to communities that have historically been underrepresented in research, as well as developed programs to help with transportation barriers. Dr. Clark adds, “We’ve also learned that people are more comfortable with doctors who share similar backgrounds, so it’s important that our investigators and clinical site teams mirror the diversity of the communities we hope to serve. We have collaborations with organizations that have been crucial in helping us reach this goal, fostering a more inclusive and representative research environment.”
Creating Your Own Path
“I’ve come to understand that the struggles I go through are not just for my own sake but to help others find their path,” says Euvon. One of his wife’s relatives began to reach out to him after his diagnosis, only to reveal that he himself was also diagnosed with prostate cancer. Euvon was able to share information about clinical trials and after speaking with his doctor, his relative was also able to participate in a clinical trial as well.
“If you’re considering participating in a clinical trial or have questions about it, the best first step is to talk to your healthcare provider. They can provide you with detailed information about how clinical trials work, the specific trials that might be relevant to you, and what you can expect from participation,” says Dr. Clark. “Your doctor can also help you understand the potential benefits and risks and guide you through the process of finding and enrolling in a trial that aligns with your needs.”
Deciding whether to join a clinical trial can be challenging, but you don’t have to do it alone. Click here to learn more about the importance of diversity in clinical trials and watch a panel discussion on this topic from this year’s ESSENCE Fest.







